Antibody (Suitable for clinical applications)
Sample Type: FFPE Patient Samples.
Tested Applications: IHC. Approved for In Vitro Diagnostic Procedures on FFPE tissues. For tissue collection recommendations, please see datasheet sent with product.
Application Notes
| Specification | Recommendation |
|---|---|
| Recommended Dilution (Conc) | 1:25-1:50 |
| Pretreatment | Citrate Buffer pH 6.0 |
| Incubation Parameters | 30 min at Room Temperature |
Prior to use, inspect vial for the presence of any precipitate or other unusual physical properties. These can indicate that the antibody has degraded and is no longer suitable for patient samples. Please run positive and negative controls simultaneously with all patient samples to account and control for errors in laboratory procedure. Use of methods or materials not recommended by enQuire Bio including change to dilution range and detection system should be routinely validated by the user.
Clonality: Monoclonal
Anti-Vimentin Antibody Clone: V9
Host and Isotype: Mouse IgG1, kappa
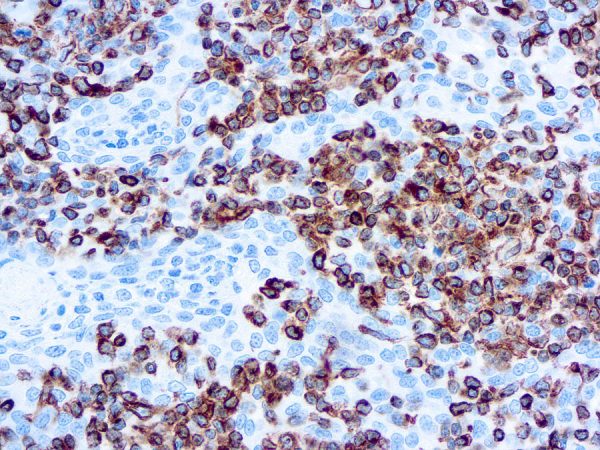
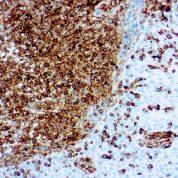
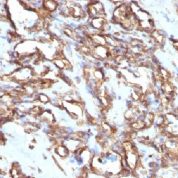
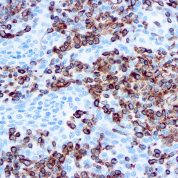
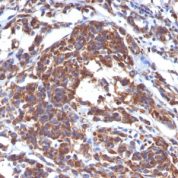
Recommended Positive Control Sample: Sarcoma, tonsil
Cellular Localization of Antibody V9 Staining: Cytoplasmic
Buffer and Stabilizer: PBS with 1% BSA and 0.05% NaN3
Antibody Concentration: Lot specific. Plese contact tech support for data.
Immunogen: BALB/C mice were injected with purified vimentin from porcine eye lens.
Storage Conditions: This antibody should be stored refrigerated (2-8°C). This product should not be used past the expiration date printed on the vial.
Vimentin Information for Pathologists
Summary:
Intermediate filament for mesenchymal tissue. Uses by pathologists Vimentin staining confirms mesenchymal origin of some tumors; may be the only positive stain and thus confirms that the tissue is capable of staining (Am J Surg Pathol 2011;35:1722). Staining may confirm mesenchymal origin, but numerous exceptions so relatively nonspecific. Help distinguish renal cell clear cell carcinoma from mimics as part of panel.Common Uses By Pathologists:
Vimentin staining confirms mesenchymal origin of some tumors; may be the only positive stain and thus confirms that the tissue is capable of staining (Am J Surg Pathol 2011;35:1722). Staining may confirm mesenchymal origin, but numerous exceptions so relatively nonspecific. Help distinguish renal cell clear cell carcinoma from mimics as part of panel. Absence of vimentin staining may confirm that fat-like or other spaces actually lack a cellular lining (Am J Surg Pathol 2011;35:1823). Distinguish endocervical and endometrial adenocarcinoma, as part of panel (Am J Surg Pathol 2010;34:915).Limitations and Warranty
This antibody is manufactured in accordance with clinical good manufacturing practices in an ISO13485:2016 certified production facility. It is intended for multiple uses including in vitro diagnostic use and research use only applications. Please see vial label for expiration date. We strive to always deliver antibodies with a shelf life of at least two years.





There are no reviews yet.