Human Anti-Carcinoembryonic Antigen / CD66 Antibody Product Attributes
Carcinoembryonic Antigen / CD66 Previously Observed Antibody Staining Patterns
Observed Subcellular, Organelle Specific Staining Data:
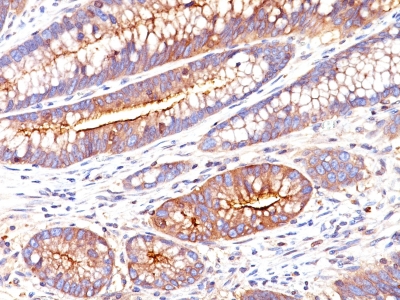
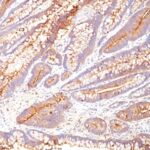
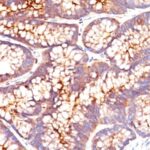
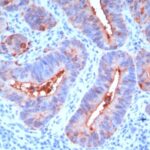
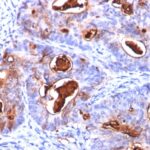
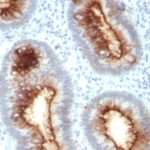
Anti-CEACAM5 antibody staining is expected to be primarily localized to the plasma membrane.
Observed Antibody Staining Data By Tissue Type:
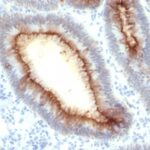
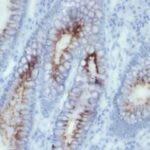
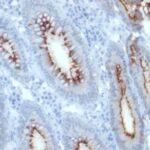
Variations in Carcinoembryonic Antigen / CD66 antibody staining intensity in immunohistochemistry on tissue sections are present across different anatomical locations. An intense signal was observed in glandular cells in the appendix, colon, rectum. More moderate antibody staining intensity was present in glandular cells in the appendix, colon, rectum. Low, but measureable presence of Carcinoembryonic Antigen / CD66 could be seen inepidermal cells in the skin, glandular cells in the gallbladder and salivary gland, hematopoietic cells in the bone marrow, hepatocytes in liver, pneumocytes in lung, respiratory epithelial cells in the nasopharynx, squamous epithelial cells in the cervix and uterine, tonsil and vagina. We were unable to detect Carcinoembryonic Antigen / CD66 in other tissues. Disease states, inflammation, and other physiological changes can have a substantial impact on antibody staining patterns. These measurements were all taken in tissues deemed normal or from patients without known disease.
Observed Antibody Staining Data By Tissue Disease Status:
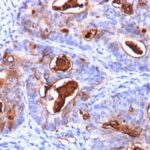
Tissues from cancer patients, for instance, have their own distinct pattern of Carcinoembryonic Antigen / CD66 expression as measured by anti-Carcinoembryonic Antigen / CD66 antibody immunohistochemical staining. The average level of expression by tumor is summarized in the table below. The variability row represents patient to patient variability in IHC staining.
| Sample Type | breast cancer | carcinoid | cervical cancer | colorectal cancer | endometrial cancer | glioma | head and neck cancer | liver cancer | lung cancer | lymphoma | melanoma | ovarian cancer | pancreatic cancer | prostate cancer | renal cancer | skin cancer | stomach cancer | testicular cancer | thyroid cancer | urothelial cancer |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal Intensity | – | – | ++ | +++ | + | – | + | – | ++ | – | – | – | ++ | – | – | – | +++ | – | – | + |
| CEACAM5 Variability | + | + | +++ | ++ | ++ | + | ++ | ++ | ++ | + | + | ++ | +++ | + | + | ++ | ++ | + | + | +++ |
| Carcinoembryonic Antigen / CD66 General Information | |
|---|---|
| Alternate Names | |
| Carcinoembryonic antigen-related cell adhesion molecule 5, CEACAM5, CD66e, Cluster of Differentiation 66e, | |
| Molecular Weight | |
| 80-200kDa | |
| Chromosomal Location | |
| 19q13.2 | |
| Curated Database and Bioinformatic Data | |
| Gene Symbol | CEACAM5 |
| Entrez Gene ID | 1048, 634 |
| Ensemble Gene ID | ENSG00000105388, ENSG00000079385 |
| RefSeq Protein Accession(s) | XP_016881634, XP_016881635, NP_001278413, NP_004354, XP_005258470, NP_001295327, XP_011524624, NP_001020083, NP_001192273, NP_001171744, NP_001703, NP_001171742, NP_001171745, XP_011525508 |
| RefSeq mRNA Accession(s) | XM_017026145, XM_017026146, XM_011526322, NM_001291484, XM_005258413, NM_001308398, NM_004363, NM_001184815, NM_001184816, NM_001205344, NM_001024912, XM_011527206 NM_001184813, NM_001712 |
| RefSeq Genomic Accession(s) | NC_000019, NC_018930NC_018930, NC_000019, NG_029051 |
| UniProt ID(s) | P06731, Q53G30, A0A024R0K5, 13688, Q3KRG8 |
| UniGene ID(s) | P06731, Q53G30, A0A024R0K5, 13688, Q3KRG8 |
| HGNC ID(s) | 1817, 1814 |
| Cosmic ID(s) | CEACAM5, CEACAM1 |
| KEGG Gene ID(s) | hsa:1048, hsa:634 |
| PharmGKB ID(s) | PA26361, PA26358 |
| General Description of Carcinoembryonic Antigen / CD66. | |
| This antibody recognizes proteins of 80-200kDa, identified as different members of CEA family. CEA is synthesized during development in the fetal gut, is re-expressed in increased amounts in intestinal carcinomas, several other tumors. This MAb does not react with nonspecific cross-reacting antigen (NCA), with human polymorphonuclear leucocytes. It shows no reaction with a variety of normal tissues, is suitable for staining of formalin/paraffin tissues. CEA is not found in benign glands, stroma, or malignant prostatic cells. Antibody to CEA is useful in detecting early foci of gastric carcinoma, in distinguishing pulmonary adenocarcinomas (60-70% are CEA+) from pleural mesotheliomas (rarely or weakly CEA+). Anti-CEA positivity is seen in adenocarcinomas from the lung, colon, stomach, esophagus, pancreas, gallbadder, urachus, salivary gland, ovary,, endocervix. | |




There are no reviews yet.