Human, Monkey, Goat, Rabbit, Rat, and Mouse Anti-Retinol Binding Protein-1 Antibody Product Attributes
Retinol Binding Protein-1 Previously Observed Antibody Staining Patterns
Observed Subcellular, Organelle Specific Staining Data:
Anti-RBP1 antibody staining is expected to be primarily localized to the cytosol and nucleoplasm.
Observed Antibody Staining Data By Tissue Type:
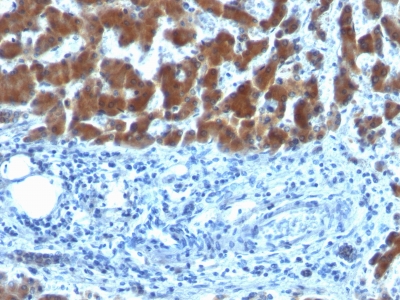
Variations in Retinol Binding Protein-1 antibody staining intensity in immunohistochemistry on tissue sections are present across different anatomical locations. An intense signal was observed in Leydig cells in the testis. More moderate antibody staining intensity was present in Leydig cells in the testis. Low, but measureable presence of Retinol Binding Protein-1 could be seen inglandular cells in the breast, glial cells in the caudate nucleus, cells in the molecular layer in cerebellum, glandular cells in the cervix, uterine, cells in the endometrial stroma in endometrium, glandular cells in the gallbladder, glial cells in the hippocampus, cells in the tubules in kidney and cells in the seminiferous ducts in testis. We were unable to detect Retinol Binding Protein-1 in other tissues. Disease states, inflammation, and other physiological changes can have a substantial impact on antibody staining patterns. These measurements were all taken in tissues deemed normal or from patients without known disease.
Observed Antibody Staining Data By Tissue Disease Status:
Tissues from cancer patients, for instance, have their own distinct pattern of Retinol Binding Protein-1 expression as measured by anti-Retinol Binding Protein-1 antibody immunohistochemical staining. The average level of expression by tumor is summarized in the table below. The variability row represents patient to patient variability in IHC staining.
| Sample Type | breast cancer | carcinoid | cervical cancer | colorectal cancer | endometrial cancer | glioma | head and neck cancer | liver cancer | lung cancer | lymphoma | melanoma | ovarian cancer | pancreatic cancer | prostate cancer | renal cancer | skin cancer | stomach cancer | testicular cancer | thyroid cancer | urothelial cancer |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal Intensity | – | – | – | + | – | – | + | – | – | – | – | – | – | – | – | – | – | – | – | – |
| RBP1 Variability | + | ++ | + | ++ | ++ | ++ | ++ | + | ++ | + | + | ++ | ++ | + | + | ++ | + | ++ | + | ++ |
| Retinol Binding Protein-1 General Information | |
|---|---|
| Alternate Names | |
| Retinol binding protein 1, cellular RBP1, RBP1 | |
| Molecular Weight | |
| 21-25kDa | |
| Chromosomal Location | |
| 10q23.33 | |
| Curated Database and Bioinformatic Data | |
| Gene Symbol | RBP1 |
| Entrez Gene ID | 5947, 5948, 5950 |
| Ensemble Gene ID | ENSG00000114115, ENSG00000114113, ENSG00000138207 |
| RefSeq Protein Accession(s) | NP_001124464, NP_002890, NP_001124465, NP_004155, NP_001310446, NP_001310447, NP_006735 |
| RefSeq mRNA Accession(s) | NM_002899, NM_001130993, NM_001130992, NM_004164, NM_001323517, NM_001323518, NM_006744, |
| RefSeq Genomic Accession(s) | NC_018914, NG_047073, NC_000003, NC_000003, NC_018914NC_000010, NC_018921, NG_009104 |
| UniProt ID(s) | A0A0A0MQT0, P09455, P50120, Q5VY30, P02753 |
| UniGene ID(s) | A0A0A0MQT0, P09455, P50120, Q5VY30, P02753 |
| HGNC ID(s) | 9919, 9920, 9922 |
| Cosmic ID(s) | RBP1, RBP2, RBP4 |
| KEGG Gene ID(s) | hsa:5947, hsa:5948, hsa:5950 |
| PharmGKB ID(s) | PA34286, PA34287, PA34289 |
| General Description of Retinol Binding Protein-1. | |
| Recognizes a protein of 21kDa-25kDa, identified as retinol binding protein (RBP). Its epitope localizes between aa 74-182 of human RBP. This MAb recognizes reduced, carboxy-methylated RBP (RCM-RBP) as well as the circulatory RBP but not the native RBP, thereby suggesting that its epitope becomes accessible either on unfolding or upon binding of RBP to transthyretin (prealbumin). RBP is responsible for distributing retinol from the retinoid stores in the liver to the various target tissues. Once secreted into the blood with bound retinol, the vitamin carrier circulates complexed with transthyretin prior to vitamin delivery at the plasma membrane through a receptor-mediated mechanism. | |



There are no reviews yet.